UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (date of earliest event reported): November 17, 2015
vTv Therapeutics Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-37524 | 47-3916571 | ||
| (State or other jurisdiction of incorporation) |
(Commission File No.) |
(IRS Employer Identification No.) |
4170 Mendenhall Oaks Pkwy
High Point, NC 27265
(Address of principal executive offices)
(336) 841-0300
(Registrant’s telephone number, including area code)
NOT APPLICABLE
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01 | Regulation FD Disclosure |
On November 17, 2015, management of vTv Therapeutics Inc. (the “Company”) gave a slide presentation to certain existing and potential investors. The presentation materials are attached hereto as Exhibit 99.1 and incorporated herein by reference. These materials may also be used by the Company at one or more subsequent presentations with investors.
The information contained in the attached presentation materials is summary information that is intended to be considered in the context of the Company’s Securities and Exchange Commission filings and other public announcements. The Company undertakes no duty or obligation to publicly update or revise this information, except as required by law.
The information in this report (including Exhibit 99.1) shall not be deemed to be “filed” for purposes of Section 18, of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Investor Presentation, dated November 17, 2015. | |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| VTV THERAPEUTICS INC. | ||
| By: | /s/ Rudy C. Howard | |
| Name: | Rudy C. Howard | |
| Title: | Executive Vice President and Chief Financial Officer | |
Dated: November 17, 2015
3
EXHIBIT INDEX
| Exhibit No. |
Description | |
| 99.1 | Investor Presentation, dated November 17, 2015. | |
4

TREATMENTS FOR THE ADVANCEMENT OF LIFE INVESTOR PRESENTATION Exhibit 99.1

Disclaimer The statements made in this presentation may include forward-looking statements regarding the Alzheimer’s disease and diabetes market, the future operations, opportunities or financial performance of vTv Therapeutics Inc. Although we believe that the expectations contained in this presentation are reasonable, these forward-looking statements are only estimations based upon the information available to vTv Therapeutics Inc. as of the date of this presentation. Except as required by law, we expressly disclaim any responsibility to publicly update or revise our forward-looking statements, whether as a result of new information, future events or otherwise. Thus, the forward-looking statements herein involve known and unknown risks and uncertainties and other important factors such that actual future operations, opportunities or financial performance may differ materially from these forward-looking statements. For a more detailed discussion of our risks, see the Risk Factors section in our prospectus filed with the SEC and our other filings with the SEC, including our most recent Quarterly Report on Form 10-Q.Undue reliance should not be placed on forward looking statements, which speak only as of the date hereof. All forward-looking statements contained herein are qualified in their entirety by the foregoing cautionary statements.

vTv Highlights- Addressing Huge Needs in Huge Markets Phase 2b data showed robust, long-lasting effect on cognitive and functional decline 3+ points on ADAS-Cog Potential benefits on CDR-SB, secondary endpoints (ADCS-ADL, MMSE, NPI) Two oral, small molecule candidates in Phase 2 studies First-in-class, Liver-selective GKA offers new treatment paradigm GLP-1r agonist with best-in-class potential Azeliragon Phase 3 AD trial has SPA, Fast Track status Key data readouts in 2016 for both diabetes candidates, 2017/2018 for Alzheimer’s Phase 3 study UNPRECEDENTED EFFICACY IN ALZHEIMER’S DISEASE DISRUPTIVE POTENTIAL IN DIABETES POSITIONED FOR CLINICAL SUCCESS

PROGRAM PRECLINICAL PHASE 1 PHASE 2 PHASE 3 STATUS EXPECTED TOPLINE DATA MILESTONES Alzheimer’s Disease Azeliragon (TTP488): RAGE Antagonist Phase 3 enrolling Late 2017/Early 2018 (Sub-study A) Mid 2018 (Sub-study B) Type 2 Diabetes TTP399: Glucokinase Activator Phase 2b enrolling Mid 2016 TTP273: Oral GLP-1r Agonist Initiation of Phase 2 trial expected in early 2016 Late 2016 Late-Stage Pipeline

Azeliragon: Late-Stage Alzheimer’s Disease Candidate Successful 2b study showed robust, long-acting effect on cognitive and functional decline; trumps other drugs in development Met primary endpoint: +3.1 points on ADAS-Cog in mild-to-moderate population Greater efficacy in mild patients, +4.0 points on ADAS-Cog Benefits on CDR-SB, all other secondary endpoints Significant reduction in incidence of psychiatric events (ex: anxiety) Novel MOA inhibits Receptor for Advanced Glycation Endproducts (RAGE) with multifactorial affects on AD etiology Impacts Aβ, tau phosphorylation, chronic inflammation, vascular dysfunction, metabolic dysregulation, and neurotoxicity Oral, once-daily therapy that would be used in combination with other agents STEADFAST Study in mild patients well-positioned for success with 80 sites enrolling SPA, Fast Track status; key data readout in late 2017/ early 2018

TTP399: First-in-Class Glucokinase Activator TTP399: Potential to transform oral diabetes market Novel mechanism of action: TTP399 is a liver-selective Glucokinase Activator (GKA) that offers a new paradigm for the treatment of type 2 and type 1 diabetes TTP399 demonstrated safety and efficacy clinical and preclinical studies Expected competitive edge vs. current non-insulin therapies: Higher efficacy Normalization of HbA1c even in “well controlled” subjects without hypoglycemia No contraindication for renal impairment No risk of pancreatitis Treating the underlying cause of the disease Phase 2b (AGATA) study expected readout mid 2016

TTP273: Best-in-Class Small Molecule GLP-1r Agonist Small molecule GLP-1r agonist Non-peptide, orally bioavailable TTP273 demonstrated safety and efficacy in clinical and preclinical studies Expected competitive edge vs. current GLP1 analogues : Superior tolerability Low incidence of GI AEs No antibody formation Ideal for combination with existing oral agents (including fixed-dose combinations) Convenience/Compliance Phase 2 (LOGRA) study expected readout late 2016

ALZHEIMER’S DISEASE PROGRAM AZELIRAGON Targeting RAGE (Antagonist of the Receptor for Advanced Glycation Endproducts)

RAGE Inhibition: A Multifactorial Target vTv owns exclusive rights to RAGE antagonists in AD until 2020 Azeliragon IP until 2029 Select RAGE Ligands: • Advanced Glycation Endproducts (AGEs) • HMGB1 • Aβ • S100 Proteins Azeliragon inhibits RAGE SIGNAL OFF SIGNAL ON • Aβ Production (β, γ secretase) • Aβ Transport • Amyloid Deposition • Vascular Dysfunction • Metabolic Dysregulation • Tau Phosphorylation and Aggregation • Cytokine Release and Chronic Inflammation Neurotoxicity and Synaptic Loss Alzheimer’s Disease and Degenerative Brain Disease RAGE Azeliragon

Evidence in Human Studies Evidence in Animal Studies Strong Clinical and Preclinical Rationale for RAGE in AD RAGE protein is increased in autopsied AD brains compared to non-demented controls.(1) Increases in RAGE protein and percentage of RAGE-expressing microglia parallel disease severity. (1) RAGE expression localizes particularly to the hippocampus.(2) Patients with AD and diabetes show increased hippocampal immuno-staining for RAGE protein.(3) RAGE knockout mice(4)(5): Resist plaque formation Are viable and display normal reproductive fitness without any striking phenotype Over-expressing RAGE transgenic mice(6): Show increased plaque formation Display abnormalities in spatial learning/memory accompanied by altered activation of markers of synaptic plasticity and exaggerated neuropathologic findings Dominant negative RAGE transgenic mice(6): Resist plaque formation Display preservation of spatial learning/memory and diminished neuropathologic changes (1) Lue L-F. Curr.Drug Targets CNS Neurol.Disord. 2005 Jun;4 (3):249-66 • (2) Jeynes B. Curr. Alzheimer Res.2008 Oct;5(5):432-7• (3) Valente T. Neurobiology of Disease 37 (2010) 67–76 (4) Deane R et al. Nature Medicine 2003 Jul;9(7):907-13 • (5) J Clin Invest. 2003 Apr;111(7):959-72. • (6) Arancio O et al. EMBO Journal (2004) 23, 4096-4105
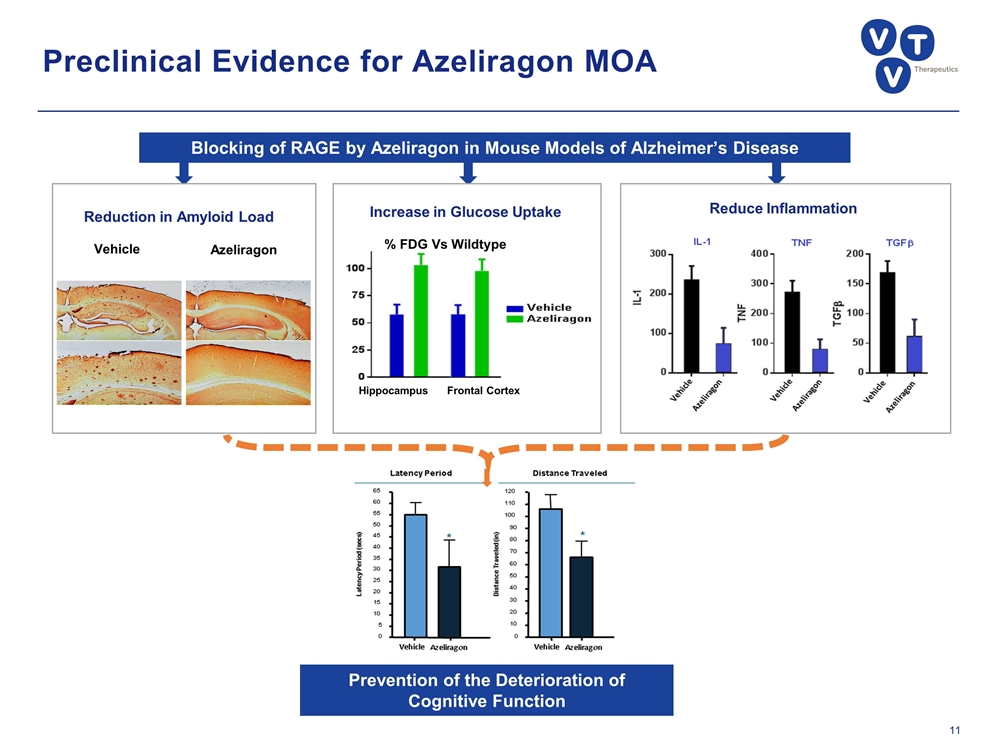
Preclinical Evidence for Azeliragon MOA Azeliragon Vehicle Reduction in Amyloid Load Increase in Glucose Uptake Hippocampus Frontal Cortex % FDG Vs Wildtype Reduce Inflammation Blocking of RAGE by Azeliragon in Mouse Models of Alzheimer’s Disease Prevention of the Deterioration of Cognitive Function
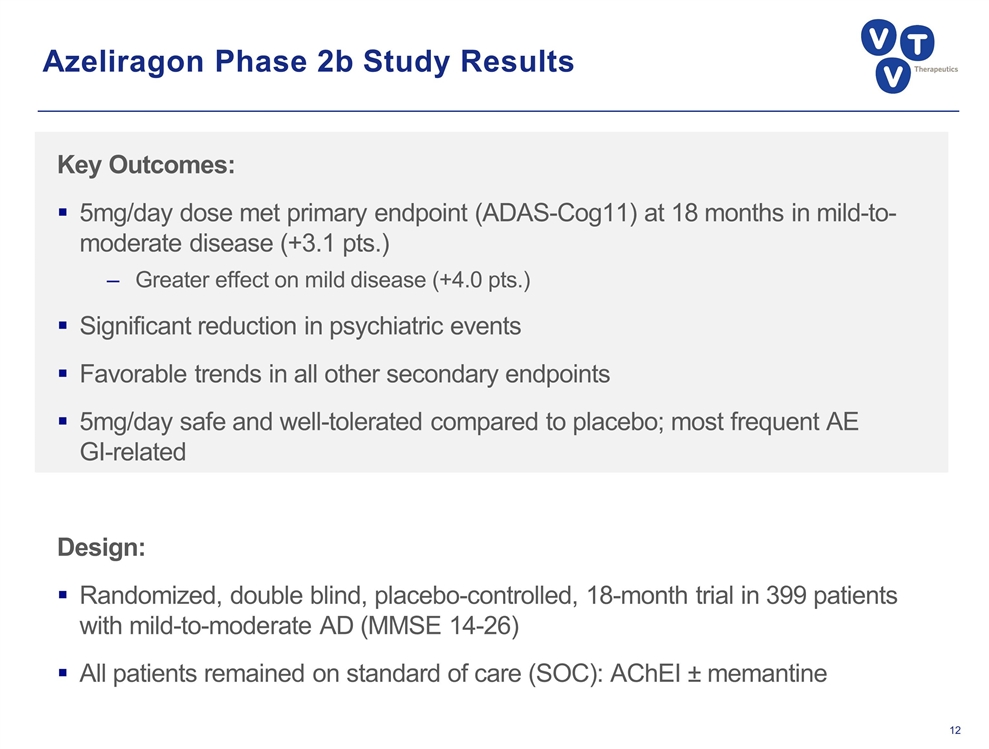
Azeliragon Phase 2b Study Results Key Outcomes: 5mg/day dose met primary endpoint (ADAS-Cog11) at 18 months in mild-to-moderate disease (+3.1 pts.) Greater effect on mild disease (+4.0 pts.) Significant reduction in psychiatric events Favorable trends in all other secondary endpoints 5mg/day safe and well-tolerated compared to placebo; most frequent AE GI-related Design: Randomized, double blind, placebo-controlled, 18-month trial in 399 patients with mild-to-moderate AD (MMSE 14-26) All patients remained on standard of care (SOC): AChEI ± memantine
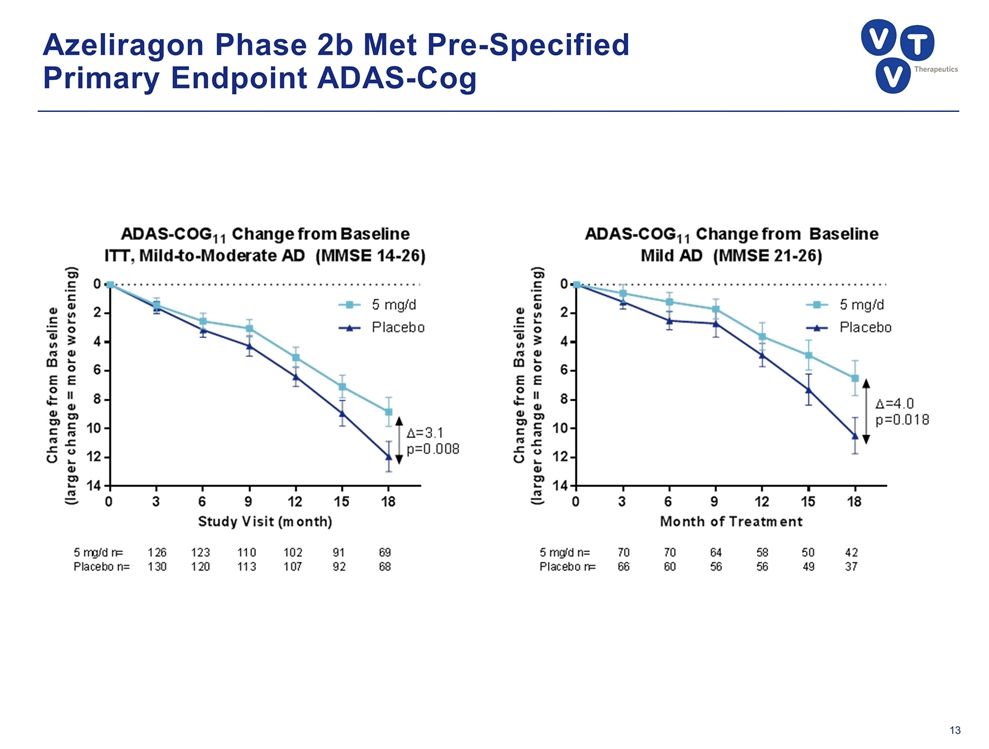
Azeliragon Phase 2b Met Pre-Specified Primary Endpoint ADAS-Cog

Azeliragon Phase 2b: Secondary Efficacy Endpoints at Month 18 FAVORABLE TRENDS IN SECONDARY ENDPOINTS EVEN THOUGH THE STUDY WAS NOT POWERED TO SHOW SECONDARY EFFICACY

Azeliragon: Significant Reduction of Psychiatric Events SAFE AND WELL-TOLERATED WITH A STATISTICALLY SIGNIFICANT REDUCTION OF PSYCHIATRIC ADVERSE EVENTS (AEs) Overall Incidence Psychiatric AEs Incidence Of Specific Psychiatric AEs p=0.037 p=0.017

Azeliragon: Unprecedented Efficacy in Alzheimer’s Disease Drug Candidate Azeliragon Phase 2b TTP488-203 (n=202) Mild Azeliragon Phase 2b TTP488-203 (n=399) Mild-Mod RVT-101 Phase 2b 0866Study1 (n= 684) Solanezumab Phase 3 EXPEDITION 12 (n= 1,012) Solanezumab Phase3 EXPEDITION 22 (n = 1,040) ADAS-Cog Benefit vs. placebo 4.0 points Week 78 3.1 points Week 78 1.65 points Week 48 0.8points Week 80 1.3 points Week 80 ADCS-ADL Benefit vs. placebo 3.2 points Week 78 1.4 points Week 78 1.9 points Week 48 -0.4 points Week 80 1.6 points Week 80 CDR-SB Benefit vs. placebo 1.0 points Week 78 0.7 points Week 78 -0.06 points Week 48 0.1 points Week 80 -0.3 points Week 80 Change in slope Yes Yes Maybe Yes Maybe Patient Population Mild AD Patients Mild-Moderate AD Patients Mild-Moderate AD Patients Mild-Moderate AD Patients Mild-Moderate AD Patients Dose 5 mg oral 5 mg oral 35 mg oral 400 mg IV 400 mg IV 1 Axovant Investor Briefing & Webcast, July 22 2015 2 Doody et al., The New England Journal of Medicine 2014, Vol. 370:4.

STEADFAST STUDY Ongoing Phase 3 Clinical Trial Under SPA Randomized, double-blind, parallel group, 18-month trial in 800 patients with mild AD 80 sites initiated and enrolling; targeting approximately 100 sites in U.S. and Canada Two independently powered sub-studies (sub-study A and sub-study B) under a single protocol: Each sub-study (n=400) has 2 arms: azeliragon 5 mg/day of azeliragon and placebo All patients will remain on standard of care (SOC): AChEI ± memantine Topline results expected in late 2017 / early 2018 for sub-study A, mid 2018 for sub-study B Co-Primary Endpoints: ADAS-COG11 and CDR-SB Secondary Endpoints: Imaging: MRI volumetric measures, FDG-PET ADCS-ADL, NPI, MMSE, COWAT, CFT, RUD, DEMQOL Biomarkers: Plasma Aβ

TYPE 2 DIABETES PROGRAMS GLUCOKINASE ACTIVATOR (TTP399) GLP-1r AGONIST (TTP273)

TTP399: First-in-Class Glucokinase Activator (GKA) Does not activate GK in the pancreas Does not interrupt the interaction of GK with the GK Regulatory Protein (GKRP) GK human mutations are a genetic validation of GK as a target for the treatment of diabetes TTP399 Is A Novel Liver Selective GKA That Targets An Underlying Cause Of The Disease Normalized Hba1c Without Causing Hypoglycemia Or Safety Concerns Has been demonstrated in clinical and preclinical studies Phase 2 (AGATA) Study Ongoing Data readout expected mid 2016

TTP399 Mechanism of Action Imbalance between glucose production (↑) and glucose utilization (↓) is a hallmark of diabetes Glucose production Glucose utilization HYPERGLYCEMIA Balance GK activation in the liver restores the balance between glucose production and glucose utilization NORMOGLYCEMIA Glucose production Glucose utilization GKA (TTP399)

TTP399: Significantly Improved HbA1c After Only 6 Weeks “Well Controlled” Subjects Mean A1C at baseline ≤ 7.5% All Subjects Mean A1C at Baseline 8.2% No hypoglycemia or increase in lipids were observed during the study Even subjects “well controlled” on Metformin (HbA1c<7.5% at day1) reached target A1c<7% TTP399, at the highest dose, normalized patients HBA1c<6.5% Pre-diabetes (normal) ADA Target A1C Non-Controlled Diabetes Diabetes Progression

TTP399 vs. Oral Diabetic Treatments Effects Observed in Patients TTP 399 DPPIV SLGT2 Efficacy Lowers blood glucose Lowers HbA1c >1% X X Normalizes HbA1c (<6.5%) X X Weight loss ? X Effective at all stages of Type 2 Diabetes X Safety No risk of ketoacidosis X No risk of genitourinary infections X No hypoglycemia *2014 Sales ($M) NA 8,800 370 High Efficacy Low Efficacy Low Side Effects High Side Effects SGLT2 DPPIV Insulin GLP-1r TTP399 *Decision Resources, Type 2 Diabetes, September 2014.

Currently Enrolling Phase 2b Study Topline Results Expected Mid 2016 A 6 month, randomized, double-blind, placebo- and active-controlled, parallel group trial in 180 type 2 diabetics on stable doses of metformin 21 sites in the United States; Principal Investigator Dr. Adrian Vella (Mayo Clinic) 7.5-9.5% baseline HbA1c Arms: Placebo TTP399 400mg once daily TTP399 800mg once daily Active comparator (sitagliptin / DPP-4 inhibitor) Primary endpoint: Change from baseline in HbA1c at 6 months Secondary endpoints: Subject achievement of HbA1c <7% at 6 months Subject achievement of HbA1c <6.5% at 6 months (normalizing HBA1c) Body weight Plasma glucose, lipids insulin, lactate, C-peptide, glucagon, GLP

TTP273: Designed to Supplant Blockbuster GLP-1 Analogues Best-in-class, Oral, Small Molecule Glp-1r Agonist Demonstrated Efficacy With Superior Tolerability Than The Peptide GLP-1 Analogues No GI side effects Phase 2 (LOGRA) Study Scheduled To Start 1Q 2016 Data readout expected late 2016 Non-peptide, orally bioavailable Ideal for combination with existing oral agents (including fixed-dose combinations)

TTP273 Clinical Results Completed 112 patient Phase 1b trial in type 2 diabetes All doses were safe and well tolerated with no serious AEs No evidence of GI side effects TTP273 demonstrated robust effects on postprandial & fasting glucose Groups defined by daily dose: PBO, Low dose (<150mg) or High dose (≥150mg)

TTP273 vs. Best Seller GLP-1 Analogues Effects Observed in Patients TTP273 GLP-1r Agonist Victoza®* Safety No significant gastrointestinal side effects X Convenience Oral X Ideal for co-formulation with existing OADs X No need for medical device X Efficacy Lowers blood glucose Decreases HbA1c levels Weight loss **2014 sales ($M) NA 1,920 Victoza® was the biggest selling GLP-1 Analogue in 2014 **Decision Resources, Type 2 Diabetes, September 2014

TTP273: LOGRA Phase 2 Study Topline Results Expected Late 2016 Randomized, double-blind, placebo-controlled, parallel group trial in 156 type 2 diabetics on stable doses of metformin Targeting 26 sites in the United States 7.5-10 % baseline HbA1c Arms: Placebo TTP273 150mg once daily TTP273 150mg twice daily Primary endpoint: Change from baseline in HbA1c at 3 months Secondary endpoints: Body weight Plasma glucose, lipids insulin, lactate, C-peptide, glucagon, GLP Subject achievement of HbA1c <7% at 3 months Scheduled to start 1Q 2016

TREATMENTS FOR THE ADVANCEMENT OF LIFE

vTv Highlights- Addressing Huge Needs in Huge Markets Phase 2b data showed robust, long-lasting effect on cognitive and functional decline 3+ points on ADAS-Cog Potential benefits on CDR-SB, secondary endpoints (ADCS-ADL, MMSE, NPI) Two oral, small molecule candidates in Phase 2 studies First-in-class, Liver-selective GKA offers new treatment paradigm GLP-1r agonist with best-in-class potential Azeliragon Phase 3 AD trial has SPA, Fast Track status Key data readouts in 2016 for both diabetes candidates, 2017/2018 for Alzheimer’s Phase 3 study UNPRECEDENTED EFFICACY IN ALZHEIMER’S DISEASE DISRUPTIVE POTENTIAL IN DIABETES POSITIONED FOR CLINICAL SUCCESS
